

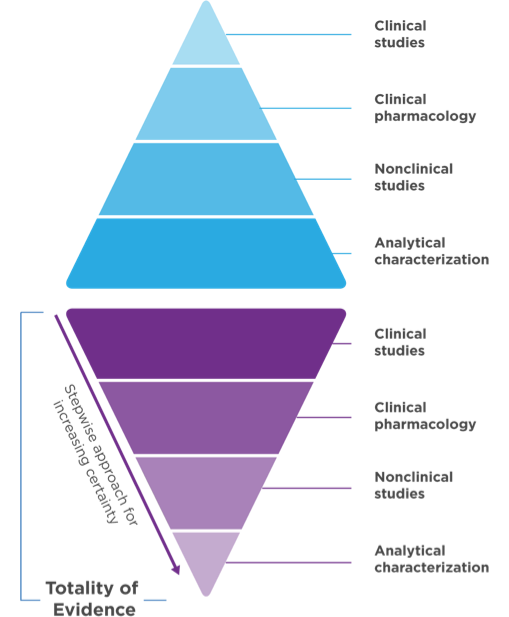
Biosimilar development1,3 Demonstrate safety, purity, and potency in one or more appropriate conditions of use for which the reference product is licensed.

References: 1. US Food and Drug Administration. Guidance for Industry: Scientific Considerations in Demonstrating Biosimilarity to a Reference Product. www.fda.gov/downloads/drugs/guidances/ucm291128.pdf. Published April 2015. Accessed December 21, 2018. 2. Conner J, Wuchterl D, Lopez M, et al. The biomanufacturing of biotechnology products. In: Shimasaki C, ed. Biotechnology Entrepreneurship: Starting, Managing, and Leading Biotech Companies. Waltham, MA: Academic Press; 2014:351-385. 3. Kozlowski S. US FDA perspectives on biosimilar biological products. Presented at: 2014 Biotechnology Technology Summit; June 13, 2014; Rockville, MD. www.ibbr.umd.edu/sites/default/files/public_page/Kozlowski%20-%20Biomanufacturing%20Summit.pdf. Accessed January 10, 2019. 4. Kresse GB. Biosimilars—science, status and strategic perspective. Eur J Pharm Biopharm. 2009;72:479-486. 5. European Medicines Agency. Guideline on Similar Biological Medicinal Products. www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/10/WC500176768.pdf. Published October 23, 2014. Accessed January 10, 2019. 6. US Federal Trade Commission. Emerging Health Care Issues: Follow-On Biologic Drug Competition. www.ftc.gov/sites/default/files/documents/reports/emerging-health-care-issues-follow-biologic-drug-competition-federal-trade-commission-report/p083901biologicsreport.pdf. Published June 2009. Accessed December 21, 2018. 7. Alten R, Cronstein BN. Clinical trial development for biosimilars. Semin Arthritis Rheum. 2015;44:S2-S8. 8. Lai Z, La Noce A. Key design considerations on comparative clinical efficacy studies for biosimilars: adalimumab as an example. RMD Open. 2016;2:e000154.