CLEAR
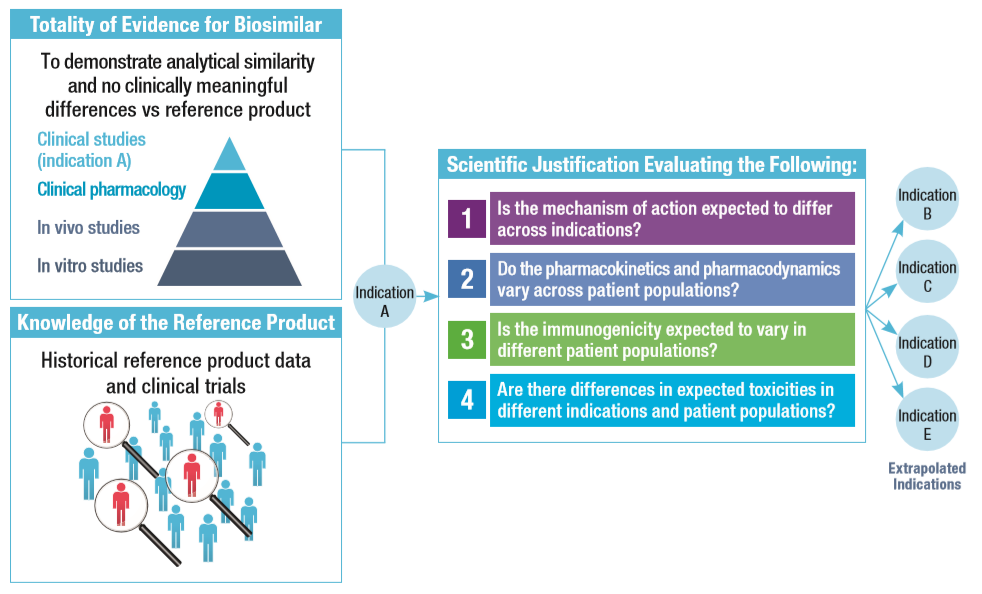




References: 1. FDA. Scientific considerations in demonstrating biosimilarity to a reference product. Guidance for industry, 2015. Available at: https://www.fda.gov/downloads/drugs/guidances/ucm291128.pdf; 2. EMA. Guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance: non-clinical and clinical issues, 2015. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/01/WC500180219.pdf; 3. Tesser JRP, et al. Biologics: Targets and Therapy 2017:11 5–11. All links accessed March 2018.